WASHINGTON – Federal officials have approved the nation’s first over-the-counter birth control pill, making it the first daily oral contraceptive to be made available in the U.S. without a prescription in a move long called for by reproductive rights organizations and leading medical associations.
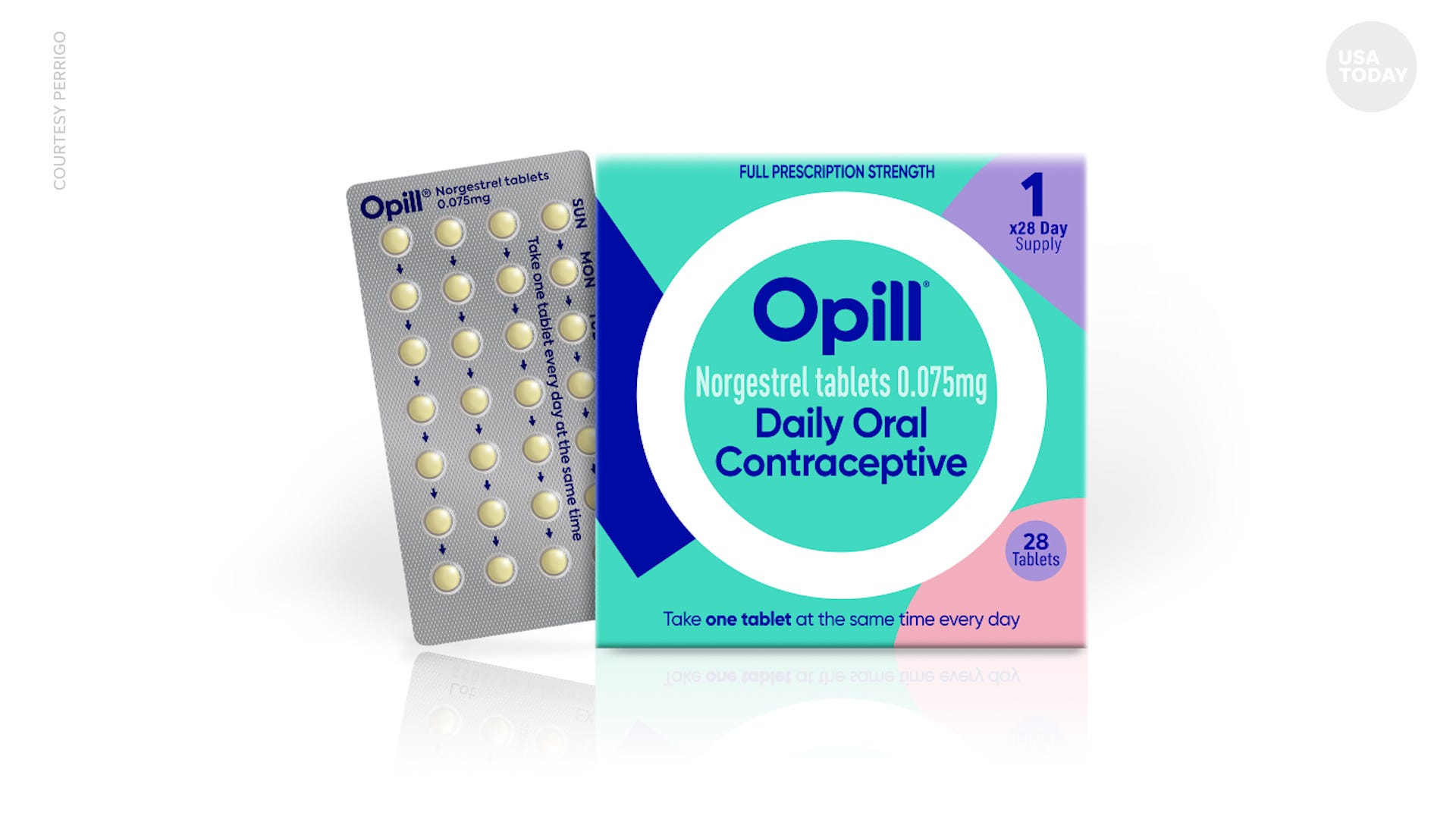
The Food and Drug Administration on Thursday signed off on Opill (norgestrel), a once-a-day tablet available by prescription since 1973 that will soon be readily available to consumers online and at drug stores, grocery stores and convenience stores.
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” said Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research.
HRA Pharma, a French drugmaker owned by Ireland pharmaceutical company Perrigo, applied for over-the-counter status last summer.
“For a product that has been available for the last 50 years, that has been used safely by millions of women, we thought it was time to make it more available,” said Frederique Welgryn, HRA’s chief strategy officer, said at the time.
Opill should hit shelves early next year
Hormone-based pills are the most common form of birth control in the U.S., used by tens of millions of women since the 1960s. Before Thursday’s announcement, all had required a prescription.
Opill is expected to be more effective than other available nonprescription methods in preventing unintended pregnancies, Cavazzoni said.
Perrigo executives said the company will spend the rest of the year manufacturing the pill and its packaging so it can be available in stores nationwide and online by early next year. There will be no age restrictions on sales.
Opill’s over-the-counter status could go a long way toward removing barriers to access since people will be able to get an oral contraceptive without the need to first see a health care provider. Teens and girls, women of color and…
Read the full article here
Leave a Reply